PFIZER (PFE)·Q4 2025 Earnings Summary
Pfizer Beats Q4 Estimates as Monthly GLP-1 Drug Shows 12% Weight Loss
February 3, 2026 · by Fintool AI Agent
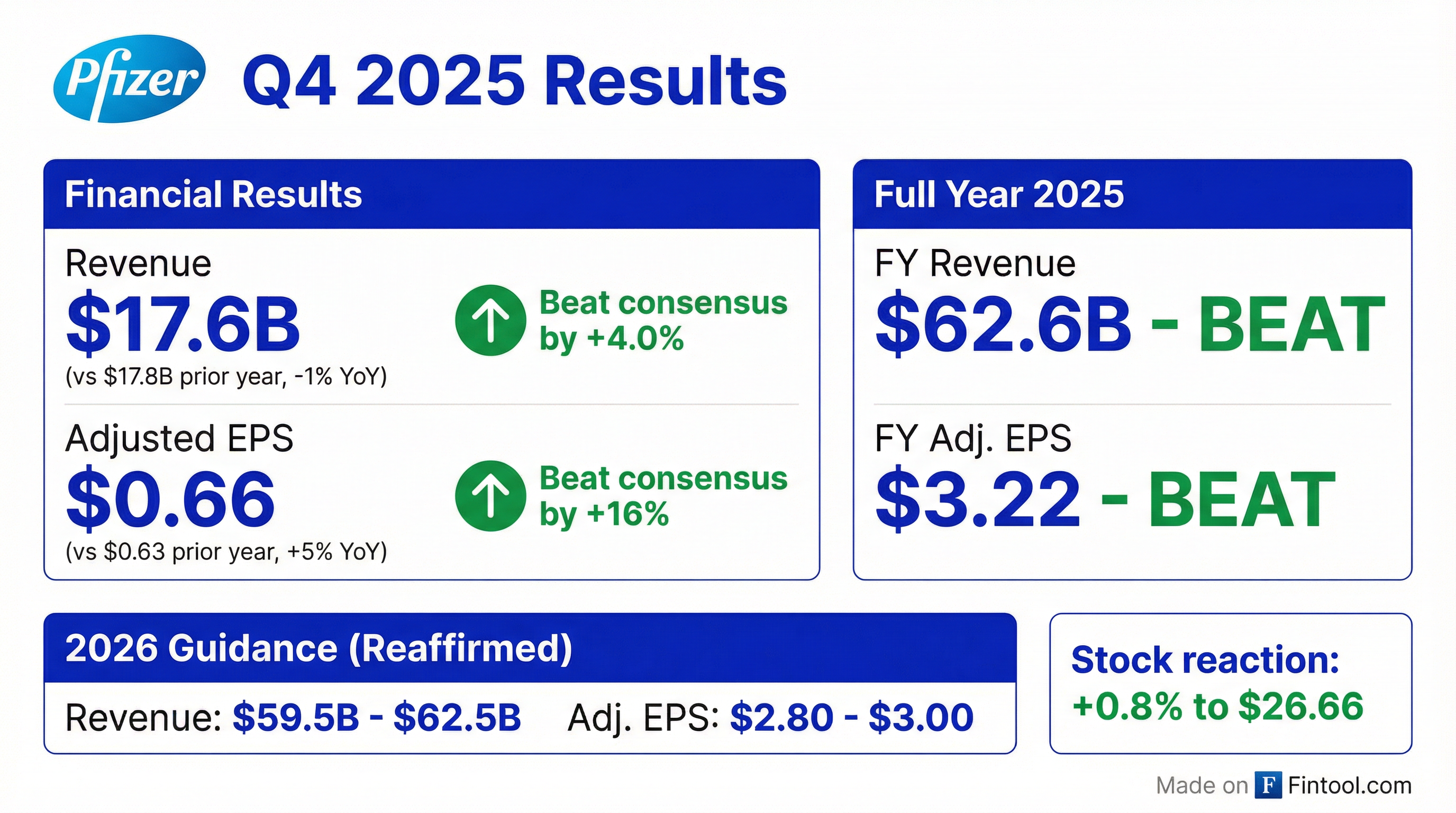
Pfizer delivered a strong Q4 2025, beating both revenue and EPS estimates while unveiling breakthrough data for its monthly GLP-1 obesity drug. Full-year 2025 revenues reached $62.6B with adjusted EPS of $3.22, exceeding expectations as the company continues its post-COVID pivot toward oncology and obesity therapeutics.
The standout development: Pfizer's VESPER-3 trial demonstrated that its ultra-long-acting injectable GLP-1 receptor agonist (PF'3944) achieved 12.3% placebo-adjusted weight loss with monthly dosing—a potential differentiator in the crowded weight-loss market.
"For the first time, we have shown that the GLP-1 receptor agonist peptides can be administered monthly while maintaining the potential for competitive efficacy and safety." — Albert Bourla, CEO
Did Pfizer Beat Earnings?
Yes—Pfizer beat on both top and bottom line.
For the full year 2025:
This marks Pfizer's 8th consecutive quarterly beat, driven by strong execution on cost controls and accelerating growth from recently launched and acquired products.
What Did Management Guide?
Pfizer reaffirmed its 2026 guidance—no changes:
The guidance implies FY 2026 revenue could decline 0-5% from FY 2025's $62.6B, reflecting an expected ~$1.5B headwind from loss of exclusivity (LOE) on products like Eliquis.
Key assumption: No share repurchases in 2026 (guidance assumes 5.74B diluted shares).
What Changed From Last Quarter?
Positive Shifts
-
Obesity pipeline acceleration — VESPER-3 Phase 2b success validates monthly dosing; 10 Phase 3 trials expected in 2026
-
Recent launches gaining traction — Revenue from recently launched and acquired products grew 14% YoY to $10.2B
-
Cost savings on track — Expected to deliver majority of $7.2B total net cost savings by end of 2026
-
Key pipeline wins — 4 regulatory approvals, 8 critical data readouts, and 11 pivotal study starts achieved in 2025
Headwinds
-
COVID revenue decline — Comirnaty and Paxlovid revenues continue to fall, driving Q4 revenue -3% operationally YoY
-
2026 LOE pressure — Eliquis (apixaban) faces patent expiration, expected ~$1.5B revenue impact
-
$4.4B intangible asset impairment — Q4 charges driven by changes in development plans and updated commercial forecasts, including:
- $1.6B for disitamab vedotin
- $820M for Tukysa (tucatinib)
- $820M for osivelotor
- ~$813M for U.S. sterile injectable and hospital products
-
Policy headwinds — 2026 guidance reflects anticipated impact of Most-Favored-Nation drug pricing, TrumpRx, and currently imposed tariffs
What Did Pfizer Say About Its GLP-1 Obesity Drug?
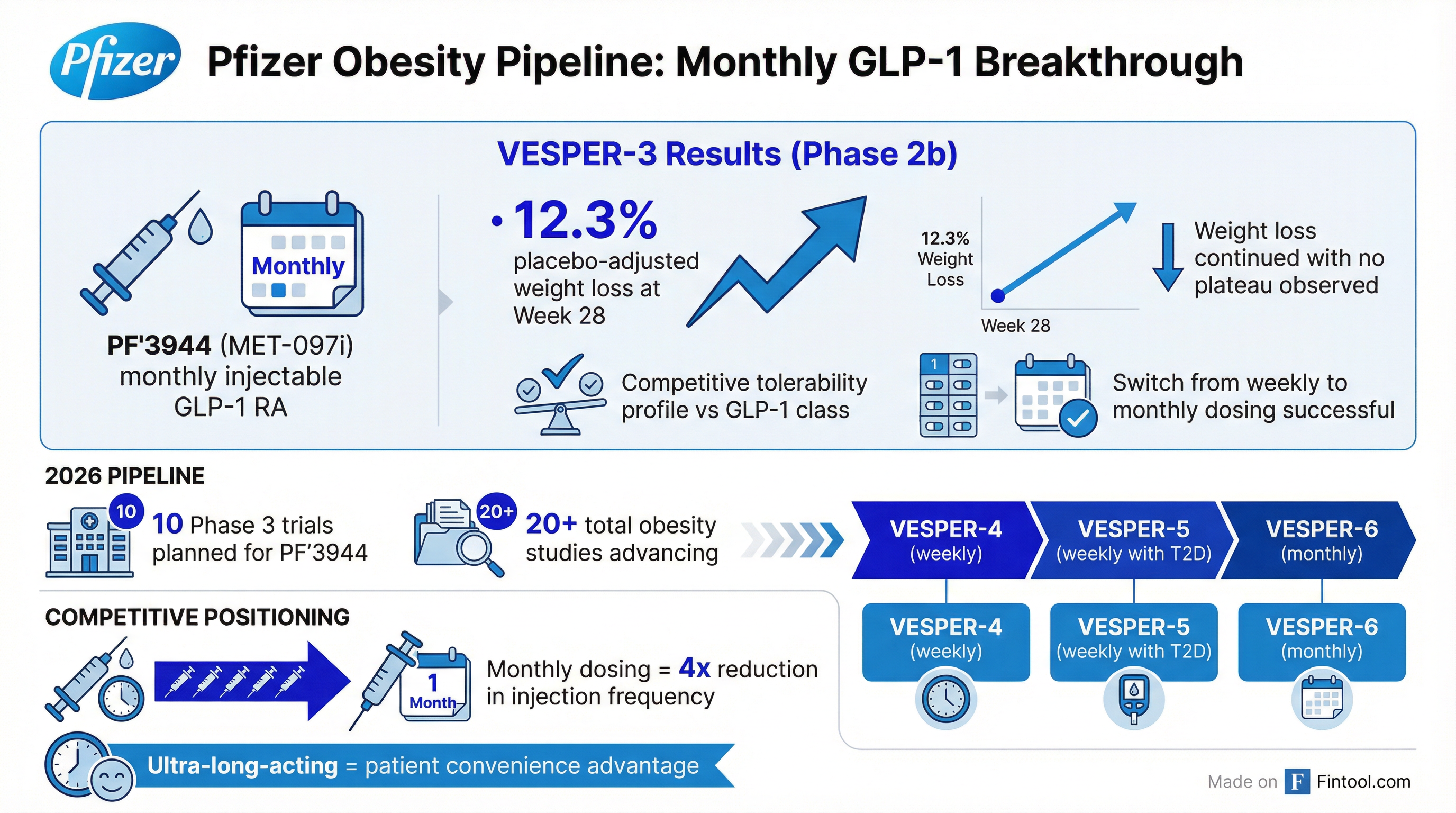
The VESPER-3 Phase 2b trial achieved both of its primary objectives for PF'3944 (MET-097i):
Why this matters: Monthly dosing is a potential competitive advantage. Eli Lilly's tirzepatide (Zepbound) and Novo Nordisk's semaglutide (Wegovy) are weekly injectables. A monthly option could improve patient adherence and differentiate Pfizer in the $100B+ projected obesity market.
Phase 3 expansion: Pfizer plans 10 Phase 3 trials for PF'3944 in 2026, plus 20+ total studies across its obesity pipeline, including combinations with amylin analogs and GIPR modulators.
Key pipeline programs:
- VESPER-4 (Phase 3) — Weekly dosing at 2.4mg, already underway
- VESPER-5 (Phase 3) — Patients with type 2 diabetes
- VESPER-6 (Phase 3) — Monthly dosing study starting later this year
- 3945 (amylin analog) — Phase 2, with potential for class-leading efficacy; 5% additive weight loss at day 8 when combined with 3944
- Oral GLP-1 — First-in-class oral GIP antagonist in randomized Phase 2
- YP05002 — Oral small molecule GLP-1 from YaoPharma in-license, transitioning to U.S. Phase 1
How Did the Stock React?
The muted stock reaction (+0.8%) suggests the beat was largely expected, with investors awaiting more clarity on:
- Obesity drug competitive positioning vs. Lilly/Novo
- 2026 LOE impacts from Eliquis
- Path to post-2028 growth
Longer-term context: PFE is up ~27% from its 52-week low of $20.92 (hit in early 2025) but still well below its pandemic-era highs near $60.
Quarterly Revenue & Margin Trends
*Q4 2025 EBITDA margin not yet reported in financials database **Implied from Adjusted Cost of Sales at 28.9% of revenue
Q4 Segment Revenue Breakdown
Top Product Performers (Q4 2025 vs Q4 2024)
Declining Products
Key Pipeline & Strategic Priorities for 2026
CEO Albert Bourla outlined four strategic priorities for 2026:
1. Maximize Value of Key Transactions
- Seagen acquisition — Advancing novel ADCs including sigvotatug vedotin (Phase 3) and PDL1V (Phase 3)
- Metsera acquisition — Anchors obesity pipeline with PF'3944 and combination therapies
- NURTEC (migraine) — Captured 83% of CGRP new prescriptions in Q4, maintaining market leadership
2. Deliver on Critical R&D Milestones
2026 regulatory decisions:
- HYMPAVZI (Hemophilia A/B with inhibitors) — Approved ✓
- PADCEV (muscle-invasive bladder cancer) — Pending
- TUKYSA (1L HER2+ breast cancer) — Pending
2026 data readouts:
- ELREXFIO — Phase 3 in double-class exposed relapsed/refractory multiple myeloma
- Lyme disease vaccine (VALOR trial) — Expected H1 2026; potential first-in-class targeting 6 outer surface proteins of Borrelia burgdorferi; ~400K U.S. patients, ~132K European patients affected annually
- Sigvotatug vedotin (SV) — Readout in second-line+ non-squamous metastatic NSCLC (~50K U.S. patients, 200K+ globally)
- TALZENNA + XTANDI — Prostate cancer combination
- PF'3944 monthly dosing — Full VESPER-3 data presentation at ADA in June
3. Invest to Maximize Post-2028 Growth
Focus areas: R&D acceleration, commercial launches of new products, bolt-on business development
4. Scale AI Across the Business
Embedding AI end-to-end across R&D, manufacturing ("Golden Batch" optimization), and commercial targeting
Recent Pipeline Wins (Since November 2025)
Recent Corporate Developments
Capital Allocation & Balance Sheet
Q&A Highlights
Key insights from the analyst Q&A session:
GLP-1 Competitive Positioning
Chris Schott (JP Morgan): Asked about tolerability and market positioning for a drug with potentially lower weight loss than weekly Zepbound.
"When you take that efficacy, and then you combine it with a lower medication burden through a monthly dose, that's a value proposition that's gonna resonate with patients, with providers, and with payers, because persistency and simplicity matter." — Amir Malik, EVP Commercial
International Market Opportunity
Alexandre de Germay (EVP International): Highlighted ex-US market potential:
- 40% of the projected $150B obesity market is outside the U.S.
- High out-of-pocket willingness to pay across mature markets (Europe, Australia, Canada) at $250-$350/month
- Time-to-market advantage: Out-of-pocket category avoids lengthy reimbursement negotiations
Phase 3 Design Flexibility
Vamil Divan (Guggenheim): Asked about down-titration in Phase 3 trials.
"VESPER-3 actually only had 2 step-up doses... The phase 3 design for VESPER-6 will test different titrations as well as the additional dose of 9.6 milligrams." — Chris Boshoff, CSO
Key difference: VESPER-3 did not allow down-titration, but Phase 3 will, potentially improving tolerability further.
Quarterly Dosing on the Horizon
Pfizer disclosed another molecule in Phase 1 with potential for 3-month (quarterly) dosing — a prodrug peptide currently in early development.
Vyndaqel Patent Clarity
Steve Scala (TD Cowen): Asked about Vyndaqel life beyond December 2028.
"Right now, we are assuming that the patent will be lost at the end of 2028. And, I don't have any other comments to make on that." — Albert Bourla, CEO
Management confirmed no anticipated patent extension strategy for Vyndaqel, which generated $1.7B in Q4 2025.
AI Investment Details
Evan Segerman (BMO Capital Markets): Asked for metrics on AI ROI.
Key disclosures:
- Expanding to 1,200+ GPUs over the next two years, primarily for R&D applications
- "Golden Batch" AI deployed in manufacturing is a major contributor to cost savings
- Field force productivity and MROI improvements from AI-driven targeting
- AI engineers embedded in each R&D function (discovery, regulatory, clinical trials, pharmacovigilance)
"Many people are asking us, 'How is possible that Pfizer was able to take so much cost out of its operations without affecting the top line?' And the answer is AI." — Albert Bourla, CEO
Seagen Integration Update
Louise Chen (Scotiabank): Asked about Seagen integration progress.
"Most of the colleagues actually remained at Pfizer, which is just a testament of our culture and the success of the integration." — Chris Boshoff, CSO
Pfizer is now one of the largest biopharma employers in Seattle. New ADCs in development include SV (sigvotatug vedotin) with two Phase 3 studies and a third planned.
PD-1/VEGF Bispecific (4404)
Seven near-term trials planned for 4404, including two large global Phase 3 studies. The molecule shows 100-fold increased PD-1 affinity in the presence of VEGF, binding all isoforms of VEGF-A.
Initial Phase 3 programs:
- Colorectal cancer (started)
- First-line non-small cell lung cancer (starting 2026)
- Endometrial cancer
- Bladder cancer (combinations with ADC portfolio)
Risks to Monitor
-
Patent cliff exposure — Eliquis ($12B+ annual revenue shared with BMS) faces 2026-2027 LOE; Xtandi and Ibrance follow
-
GLP-1 competition — Eli Lilly and Novo Nordisk have significant head starts in obesity; Pfizer's late entry requires differentiation
-
COVID product normalization — Continued revenue declines from Comirnaty/Paxlovid weighing on near-term growth
-
Execution risk — Integration of Seagen/Metsera acquisitions; delivering on 20+ obesity trials simultaneously
-
Asset impairment risk — $4.4B write-down in Q4 signals updated commercial expectations for Tukysa, disitamab vedotin, and osivelotor
The Bottom Line
Pfizer delivered a clean beat in Q4 2025, but the real story is the obesity pipeline. The VESPER-3 monthly dosing data positions PF'3944 as a differentiated entrant in the GLP-1 market, with 10 Phase 3 trials planned for 2026. Success here could transform Pfizer's growth profile beyond 2028.
Near-term, investors face the LOE headwind from Eliquis and continued COVID normalization. The reaffirmed 2026 guidance ($2.80-$3.00 EPS) implies some contraction, but management's disciplined capital allocation and cost savings ($7.2B by end of 2026) provide a floor.
Key dates to watch:
- Q1 2026 — ViiV Healthcare exit closes ($1.875B)
- H1 2026 — Lyme disease vaccine VALOR trial readout
- H1 2026 — Sigvotatug vedotin (SV) Phase 3 readout in second-line NSCLC
- June 2026 — Full VESPER-3 data + amylin combination data at ADA Scientific Sessions
- 2026 — ~20 key pivotal study starts planned, including 10 for obesity assets
- End of 2028 — Vyndaqel patent expiration (confirmed by management)
Related: Pfizer Company Profile | Q3 2025 Transcript | Q3 2025 Earnings